|
| Author |
Message |
rockies
Member
|
# Posted: 8 May 2018 20:09 - Edited by: rockies
Reply
Students built this with $100 worth of piping and a 4x8 sheet of reflective steel.
https://www.renewableenergymagazine.com/thermal/students-from-san-jose-state-universi ty-us
http://www.solaripedia.com/13/389/5541/solar_ice_maker_illustration.html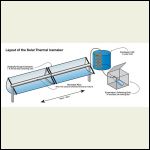
Possible Setup
| 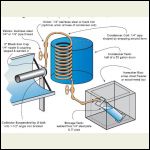
Possible Setup
|  |  |
|
|
sparky30_06
Member
|
# Posted: 11 May 2018 07:44
Reply
Wonder what refrigerant they are using? Most refrigerant needs some pretty good pressure drop over a metering device.
|
|
rockies
Member
|
# Posted: 11 May 2018 19:31
Reply
The refrigerant is an ammonia/salt mixture. The mixture can hit 200 lbs psi as it moves back and forth within the piping so the piping has to be able to withstand a lot higher pressure. I'm trying to get more precise guidelines for constructing one.
|
|
darz5150
Member
|
# Posted: 11 May 2018 21:17
Reply
https://en.m.wikipedia.org/wiki/Icyball
|
|
rockies
Member
|
# Posted: 12 May 2018 18:51
Reply
I think what is great about the San Jose version is that there is no participation required to operated the ice maker. The sun evaporates the ammonia out of the salt in the collector pipe, the ammonia gas moves into the condenser tank and then collects inside the storage tank (which is itself kept inside an insulated box).
At night the cold collector pipe naturally draws the ammonia back up through the same pipe (because of pressure differences) so it just recycles over and over.
When the sun goes down just add your plastic containers of water inside the insulated box and you'll have ice in the morning. No electricity, no moving parts.
|
|
sparky30_06
Member
|
# Posted: 14 May 2018 07:03
Reply
Had a feeling it was ammonia. Old school and very dangerous, depending on how much is present.
|
|
|

