|
| Author |
Message |
rockies
Member
|
# Posted: 1 Jun 2017 07:18pm
Reply
Propane Usage
Propane gas is measured and distributed in gallons, pounds and cubic feet, and is usually sold by the pound when dispensed into portable tanks, and sold by the gallon when weighing the tank isn’t feasible.
Propane tanks are typically filled to 80% capacity to leave room for expansion.
1 Gallon of Propane = 27 kWh (Kilowatt Hours) of electricity.
1 Gallon of Propane = 91,600 Btu’s.
1 Gallon of Propane = 4.2 pounds as a liquid at 60-degrees F.
1 Gallon of Propane = 35.97 cubic feet.
1 Pound of Propane = 21,810 Btu’s.
Â
“How long will my portable tank of propane last?†This is easy to figure out if you know the number of pounds of gas that’s in your full tank and the btu/hr demand of your burner or other gas appliances. One pound of liquid gas in your tank has 21,600 Btu/hr fuel value.
If you have a typical portable 20# tank, and if you have a typical low pressure burner gas grill, for example that is rated at 40,000 btu/hr maximum output, then you can run that burner at full blast for 10.9 hours:
(20# x 21,810 Btu/# = 436,000 Btu’s in the gas in a 20# tank)
(436,000 Btu ÷ 40,000 Btu/hr = 10.9 hrs)
|
|
NorthRick
Member
|
# Posted: 2 Jun 2017 02:41pm
Reply
You don't need those fancy equations to figure out when the propane runs out. It will run out right in the middle of grilling some expensive steaks. 
|
|
Cowracer
Member
|
# Posted: 2 Jun 2017 04:35pm - Edited by: Cowracer
Reply
Quoting: rockies One pound of liquid gas in your tank has 21,600 Btu/hr fuel value.
Only at about 55 degrees F. BTU/HR drops off dramatically with lower temperatures. At 0 deg F, you are down to 7500 BTU/hr.
Many guys on the RV forum I am on wonder why their furnace can't keep up when it starts getting cold. There just ain't enough BTUs available in from the propane tanks.
Tim
|
|
ForceFed70
Member
|
# Posted: 3 Jun 2017 12:07pm - Edited by: ForceFed70
Reply
KWh = Kilowatts per hour
Converting 1lb of propane to KWh is like saying 5280 feet = 1 mile per hour.
You could say 1lb of propane per hour = xxxxx KWh. Or you could say 1lb of propane = xxx KW for 1hr.
|
|
ForceFed70
Member
|
# Posted: 3 Jun 2017 12:54pm - Edited by: ForceFed70
Reply
Quoting: Cowracer Only at about 55 degrees F. BTU/HR drops off dramatically with lower temperatures. At 0 deg F, you are down to 7500 BTU/hr.Many guys on the RV forum I am on wonder why their furnace can't keep up when it starts getting cold. There just ain't enough BTUs available in from the propane tanks.Tim
The amount of chemical energy stored in propane is not influenced by the temperature.
Most likely the reason their RV heaters can't keep up is poor insulation and/or undersized heater. It's also possible that at low temperatures the heaters and propane system stop operating correctly.
Propane pressure is influenced by temperature. That's why we have regulators - to keep the pressure consistent despite the temp. But the energy stored does not change with temp.
|
|
Cowracer
Member
|
# Posted: 4 Jun 2017 07:57pm - Edited by: Cowracer
Reply
Quoting: ForceFed70 The amount of chemical energy stored in propane is not influenced by the temperature. Most likely the reason their RV heaters can't keep up is poor insulation and/or undersized heater. It's also possible that at low temperatures the heaters and propane system stop operating correctly. Propane pressure is influenced by temperature. That's why we have regulators - to keep the pressure consistent despite the temp. But the energy stored does not change with temp.
True, the energy in a gallon of propane does not change with temp, but we do not burn liquid propane, we let it convert to gas. This conversion requires and absorbs heat, which is why you see frost on your propane tank in the proper conditions. The rate that propane can covert to gas is totally dependent on available ambient heat. Colder=less propane liquid converted to gas.
The vaporization rate greatly affects available BTU's for use, even if actual BTU's of the fuel does not change.
Tim
|
|
Bigred292
Member
|
# Posted: 4 Jun 2017 09:35pm
Reply
Interesting info that I've been trying to use. Have a Dometic fridge , tag says "input 1500 btu/ hr"
If I were to leave my fridge on all of the time how long would a bottle last? I have 2 100 lb tanks ( I think they're 100 lbs- like chest high)
I came up with calculation of 50 days- does that sound right?
|
|
Bigred292
Member
|
# Posted: 4 Jun 2017 09:36pm
Reply
50 days per tank
|
|
|
deercula
Member
|
# Posted: 4 Jun 2017 09:47pm
Reply
Quoting: ForceFed70 It's also possible that at low temperatures the heaters and propane system stop operating correctly.
Yes, Due to starving for gas.
|
|
ForceFed70
Member
|
# Posted: 5 Jun 2017 01:00am - Edited by: ForceFed70
Reply
Quoting: Cowracer True, the energy in a gallon of propane does not change with temp, but we do not burn liquid propane, we let it convert to gas. This conversion requires and absorbs heat, which is why you see frost on your propane tank in the proper conditions. The rate that propane can covert to gas is totally dependent on available ambient heat. Colder=less propane liquid converted to gas. The vaporization rate greatly affects available BTU's for use, even if actual BTU's of the fuel does not change.
Only due to poor design. Yes, the process of turning liquid propane to gaseous does require heat. -44*F to be exact. That's the temp at which liquid propane converts. So long as the outside temp is above -44* ambient temp you can convert without adding additional heat. If your regulator is icing up and unable to deal with the cold - it's just a design problem with the heating system. A larger regulator that's better able to dissipate the cold is all you need to fix. Or dual regulators.
Long story short - your post was very misleading. Unless you are camping in temps below -40* your heater can work without issue if the system is designed properly. Propane doesn't "loose BTU's" your regulator freezes up and doesn't deliver the propane in the 1st place.
That chart you've posted is also misleading It's part of a system design with a specific regulator, etc. Place additional heat sinking within the system and you'll be able to deliver more gas without icing up. It's actually the regulator that get's cold, it's just that the tank is used as a heat sink hence why the larger the tank (larger the heat sink), the more gas it can provide before icing up.
|
|
Cowracer
Member
|
# Posted: 5 Jun 2017 09:05am
Reply
well sir. As the expert, I will defer all propane questions to you in the future.
tim
|
|
ForceFed70
Member
|
# Posted: 5 Jun 2017 12:55pm
Reply
Not an expert. Just a Canadian who's lived with and used propane in cold temperatures for many years.
You learn little tricks like removing tank covers. Using dual regulators or oversized regulators, etc.
Propane powered vehicles are fairly common in Canada. They don't loose power when it get's cold - tho creative solutions are often used like regulators that are ment to be warmed by circulating engine coolant/antifreeze through them.
|
|
NorthRick
Member
|
# Posted: 5 Jun 2017 12:59pm
Reply
What Cowracer posted is correct. Yes, you could be having regulator issues in cold temps, but even if it is functioning 100% perfect, the rate at which propane gas can come out of a given tank will drop as the temperature drops. It's a physics thing.
|
|
ForceFed70
Member
|
# Posted: 5 Jun 2017 01:17pm - Edited by: ForceFed70
Reply
Yes, given a specific tank (with a specific valve opening size) the rate of flow drops as the pressure inside the tank drops.
Have you ever opened the valve of a propane tank at -20*F? If so you'd know that even at -20* the average tank can still provide WAY MORE propane than an RV would require. The limitation is further downstream.
As mentioned, propane pressure drops with temperature. At -44*F the pressure is 0 PSI (compared to atmospheric pressure). That's the physical limit at which point the tank cannot deliver any propane. Here's a handy chart: https://www.agas.co.uk/media/2422/r290-propane-pt-chart.pdf
Propane appliances operate at a little less than 1PSI (above atmospheric pressure). So the tank must be able to supply 1PSI or more of pressure. If you look at the chart, that's at about -42*.
Almost all propane systems require more than 1 PSI to operate correctly. This is due to design limitations however, not a physical limitation. Lines are too small, regulator needs a few PSI to open, etc. In practical terms, operating propane appliances below -20* is a challenge and most systems aren't designed to work below 0* or even warmer.
From a "what's the coldest temp you could possibly use propane at" perspective - the answer is -42*F regardless of the amount of propane needed. You could deliver large amounts of gaseous propane at -42*F but it would require very large, complex, and impractical heat sinking to make it happen - but it could be done.
|
|
NorthRick
Member
|
# Posted: 5 Jun 2017 01:44pm
Reply
What you are missing is that when a liquid volatilizes, the remaining liquid drops in temperature (that's the physics part). The faster you "pull" gas out of a tank, the lower the temperature the liquid in the tank becomes. At warmer temperatures, the air surrounding the tank provides heat to the liquid propane and at some point you reach a steady state on how low the liquid temperature becomes.
Google "Jet Powered Beer Cooler" for a funny, albeit impractical, look at how someone used this fact to make beer cold.
|
|
ForceFed70
Member
|
# Posted: 5 Jun 2017 02:01pm
Reply
No, not forgetting that. Yes, the temp of the tank drops with the change in state. But that's why I mention heat sinking and design limitations, why it's a challenge to use propane below -20*F, etc.
I still stand by my assertion that your average tank will provide plenty of propane at -20*F to run an RV. It's the other components (regulator usually) that limit you 1st.
Thanks for the video! Another even simpler way to demonstrate this property/effect was done on mythbusters by using a CO2 fire extinguisher to cool beer.
|
|
Cowracer
Member
|
# Posted: 5 Jun 2017 02:19pm - Edited by: Cowracer
Reply
Quoting: ForceFed70 I still stand by my assertion that your average tank will provide plenty of propane at -20*F to run an RV. It's the other components (regulator usually) that limit you 1st.
Quoting: ForceFed70 Have you ever opened the valve of a propane tank at -20*F? If so you'd know that even at -20* the average tank can still provide WAY MORE propane than an RV would require. The limitation is further downstream.
Not if the furnace is trying to make more than 2000-3000 btu, which is barely enough to make a good bowl of soup. Its not an engineering thing, its a physics thing. Nice thing about physics is, it really don't matter if you believe it or not. You can "stand by" whatever you want. Truth is the truth, and not just an opinion of a guy who thinks he knows what he is talking about.
Tim
BTW... The cars in Canada, and most industrial forklifts that run propane use a VAPORIZER, to feed the engine, not a regulator. This vaporizer is fed LIQUID propane, and is heated to ensure that a constant supply of gaseous propane is produced to be available to the engine. And I am most certainly a guy who knows what he is talking about there.
|
|
ForceFed70
Member
|
# Posted: 5 Jun 2017 02:38pm - Edited by: ForceFed70
Reply
Quoting: Cowracer Not if the furnace is trying to make more than 2000-3000 btu, which is barely enough to make a good bowl of soup. Its not an engineering thing, its a physics thing. Nice thing about physics is, it really don't matter if you believe it or not. You can "stand by" whatever you want. Truth is the truth, and not just an opinion of a guy who thinks he knows what he is talking about.
Fair enough - but let me turn the table on you then. Please show me how you've come to this conclusion? Do you have some sort of a calculation or evidence to prove your statement of 2-3kBTU? Why can you make unfounded assertions, but I cannot?
I was trying to keep things simple with the vehicle reference. A vaporizer is essentially just a heated regulator that still needs to operate without additional heat for a period of a few minutes until the engine itself has warmed up. I even mentioned that they're warmed by the engine. The vaporizer is not attached to throttle linkage the amount of propane is regulated by pressure.
|
|
Cowracer
Member
|
# Posted: 5 Jun 2017 03:09pm
Reply
Quoting: ForceFed70 Fair enough - but let me turn the table on you then. Please show me how you've come to this conclusion? Do you have some sort of a calculation or evidence to prove your statement of 2-3kBTU? Why can you make unfounded assertions, but I cannot?
I can put up a Mollier Diagram for propane, but I think it might be a little confusing.
Tim
|
|
ForceFed70
Member
|
# Posted: 5 Jun 2017 03:26pm - Edited by: ForceFed70
Reply
Thanks. Yes, it's a little confusing. If I understand it correctly, it's showing the amount of heat required to convert liquid to gaseous propane depending on pressure and volume.
It doesn't show max BTU or anything like that tho. In fact, BTU isn't referenced at all.
|
|
Cowracer
Member
|
# Posted: 5 Jun 2017 03:42pm - Edited by: Cowracer
Reply
Quoting: ForceFed70 It doesn't show max BTU or anything like that tho. In fact, BTU isn't referenced at all
Molliers don't show BTU. The BTU of 100% propane is understood at 21,600 BTU per pound but ONLY if all of it is burnt. As we generally burn propane in gaseous (vapor) form, that 21,600 per pound is understood if we can convert the WHOLE pound into vapor.
The mollier shows a couple of different things at the same time, but one data that can be extracted from it is how much propane can be converted to vapor at varying pressures and temperatures. The sloping right-hand leg of the 'dome' is where full conversion to vapor happens. You can see a dramatic drop-off in temp when it does. (temp is the lines that are horizontal in the dome region labeled T=XX. As you can see, more energy is available to the system (Enthalpy) as temps go higher. This is NOT btu's produced by the vapor, this is just the energy total energy in the system, and THAT is the energy that will vaporize the liquid.
This might help in figuring out how to read it.
http://web.mit.edu/10.213/oldpages/f99/diagrams/preenth/index.html
Tim
|
|
ForceFed70
Member
|
# Posted: 5 Jun 2017 03:52pm
Reply
Gotcha - thank you. Yes, so now we know how many BTU's because we know the amount of energy in a KG of propane. Should have considered that.
The diagram, tells us how much heat energy is required to convert xxx BTU (based on weight of propane).
I still don't see how we can use this to say "2-3k BTU Max" tho.
Don't you need to calculate the maximum heat input from the surrounding environment? Then compare it to the graph data before you can make that assertion?
|
|
Cowracer
Member
|
# Posted: 5 Jun 2017 04:47pm - Edited by: Cowracer
Reply
Quoting: ForceFed70 I still don't see how we can use this to say "2-3k BTU Max" tho. Don't you need to calculate the maximum heat input from the surrounding environment? Then compare it to the graph data before you can make that assertion?
No because someone way smarter than me already did the all math on that for us, taking in account tank size (has an effect on thermal transfer), tank level, tank pressure, ambient temperature, vapor pressure, enthalpy of the system, etc etc etc and came up with the handy chart I showed you above.
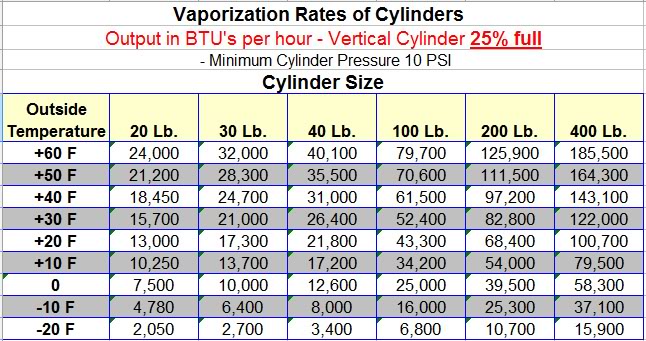
If you recall, you called this chart "Misleading" and said that it is only for a specific regulator. I hope you can see now that it is not misleading. The downstream equipment (regulator, heat-sinks, etc) do not matter a whit in how much liquid CAN BE converted to vapor in the tank, and ergo, how many usable BTU's are provided. Note that I said "in the tank". Which is how 100% of consumers use portable propane bottles. You want to take liquid out of the tank and vaporize it in a heated area or heated vaporizer, you are talking a whole different ball of wax, as you have artificially increased the ambient temp for the vaporization process.
So at the "-20 degrees" that you mentioned, the BTU's available to the furnace (remember, its fed vapor, and we are talking about the ability to vaporize propane at low temps) is shown in the chart as 2050. I ranged from 2000 to 3000 just to cover my bases
Tim
|
|
ForceFed70
Member
|
# Posted: 5 Jun 2017 05:05pm - Edited by: ForceFed70
Reply
This doesn't tie the 2 together. Now we've ditched the graph completely and went back to the chart. It's based on unknown calculations by another party and cannot be verified.
I do believe that chart to be accurate for a typical tank and regulator combo. I called it misleading because it was presented as a maximum. When in reality it represents a typical or "worst case scenario". More BTU can be obtained if you improve the heat sinking properties. EG. Larger or Dual regulators, improved airflow, finned tanks, submerged in an antifreeze bath, etc. That's the point I was trying to make - It's possible to get more. It may not be easy or practical, but it's possible.
|
|
Cowracer
Member
|
# Posted: 5 Jun 2017 05:51pm
Reply
I give up. You win. I went a long way to show the science and math, but I guess that means nothing to someone who's main argument is "I do believe..."
Ill just let ASHRE know that they can close up shop, no need for engineers now that everything is so simple.
Tim
|
|
ForceFed70
Member
|
# Posted: 5 Jun 2017 06:12pm
Reply
OK, if that's the way you want to go.
You seem to understand the core of the problem "It gets cold, limiting output". Why not understand that "removing some of the cold via better heat sinking would up the limit"?
Why do you believe I'm saying the folks at ASHRE are wrong? I've never made that claim.
Quoting: ForceFed70 I do believe that chart to be accurate for a typical tank and regulator combo
|
|
|

